These memorable quotes are taken directly from my students’ lab reports, tests, quizzes, and homework assignments.
Acquiring the acceleration was detrimental to being able to seek out the total net force.
Each checkpoint was measured using a trembling wheel that was 1/3 of the floor apart from each other.
When it builds up potential energy due to us holding it for a long time and when we let it go the velocity builds up and kinetic energy is made then when it reaches the end it builds up less potential energy since it goes back to kinetic energy quickly but since it was a quick transition there is less of it and that’s why physics says that the bowling ball won’t come back and hit your face breaking it and leaving you in the hospital unless you didn’t follow the instruction then that’s on you but physics says that it should come back not fully in its kinetic energy when we let it go the first time.
When the cart is moving physics says that when weight is added the cart will have extra velocity but after a little while science also says that it will stop sooner.
Mr. Bigler, with the consent of [name], has stolen some of [name]’s electrons from her hair and is using those electrons to stick a balloon to the wall.
It would have been better if velocity wasn’t involved because due to uncertainty, our answers are not exact and may have some errors. [Note that determining velocity was the objective of the experiment.]
The reason why we used multiple equations was because there were a lot of variables missing.
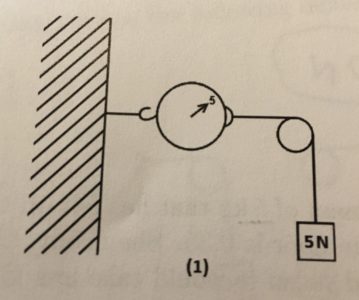
[Newton’s] Law I can be illustrated by the third demo since you can see there are no continuation at certain rest while there is motion remaining.
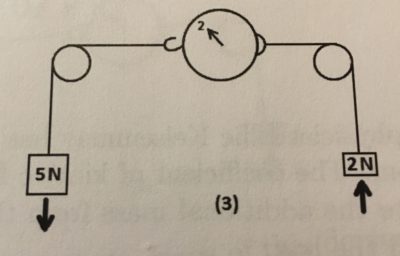
The 1st demo represents [Newton’s first] law because as the object is in motion being pulled by the force of the 5N weights, but is also at rest due to the wall on the left.
Using these factors we are going to determine the difference in mass of a candle using these variables. Using these will help show the effect the procedure had on the candle.
This causes the behavior of remaining unharmed because as we increase the amount of area, the total amount of pressure decreases causing the expected behavior of remaining unharmed.
This demonstrates that conservation of energy cannot be created or destroyed without the act of an outside force.
The negative charge is sucked up by the positive charge because opposites attract.
dh is 9.68 seconds as the velocity Mr. Bigler walk while the egg is in the air.
The ball would make impact with the floor immediately after being dropped causing it to bounce in an awkward motion.
Also, a 90° angle will have more height at a 45° angle.
The purpose of the experiment is that you create a experiment that will last 10 seconds which means you have to create a experiment that lasts that long.
For the metal you need a which is heat which you get from to heat up the water. Then calculate to find Q/C.
This activity illustrates the relevant principle of physics by involving some of the units or sign or symbol used to represent units in physics. The equation was created or predicted according to some of the units the question or activity has in it and the acceleration due to gravity was involved in the equation because when we was doing the activity acceleration due to gravity occurred.
This activity illustrated the relevant physics principle because it showed us that physics has an answer to everything.
By discovering the value of Fm, it showed us how many “N” it took to do the things it can do.
The energy from the ball travels through a person’s body which is wicked awesome.
The momentum and the velocity totaled out together will help you find the momentum.
So the defendant variable would be 9.8 × 5.4 which equals 52.92, that is the solution.
An electric motor is an electromagnet that spins because of its continuously switching attraction and repulsion to the magnetic field produced by a separate set of permanent markers.
The resistor is used to lighten the load of the LED, so I didn’t burn out. (Note: this was hand-written, and the “I” was capitalized.)
Example of Newton’s Second Law: the force of acceleration of a car when it moves (accelerates) a moving car.
An example of Newton’s Third Law is that if I flick my friend on the head (which is not nice), I am committing an action and her reaction will be to turn her head and say “Ow!”
If it were positive, particle would stay positive. If it were negative it would move negatively and if it were neutral it would stay positive.
In conclusion, if there was only one nail, one will get a big boo-boo by sitting on it, since it cannot support our area.
Ice is a solid liquid. Therefore, if ice was put in water the ice will melt which will make water expand and overflow.
To find water use Q = mCΔT. Use results to find Q = mCΔT for mostly metal.
According to the chart given to us, the unknown metal is closest to granite.
Take temperature temperature and turn on gas.
Measure the equipment and metals before it is heated. Also take the temperature before too. Then we will identify the different varribles like the temperature of the water then label them. Finally, measure the different varribles for each metal.
Use thongs to take the beaker off the burner and to take out the metal.
Have the person get on the bike. Tell the person to paddle while someone else keeps the time.
Experimental Plan: To explicitly mention what I need to measure and what I will calculate from it.
So, if asteroid #1 collides with asteroid #2, the crash of the collidance of the two asteroids are moving. Thus they will continue to stay that way (moving).
When she sticks her leg out she slows down a little, because it messed with the force.
The density was found after doing everything.
Even though the color changed it was still not in the form of an electron.
The objective is to drop an egg on Mr. Bigler’s head using calculators.
In the lab of chemical reactivity goals were reached…Various tools were used to reach the goals and during different times through the experiment results were recorded so that when the lab was concluded trends could be recognized. Results were reached through several steps.
When the electrons begin to come back down to lower energy states they give off protons.
This interesting experiment was done to find out the color of different electrons.
Before this experiment was performed, photons were considered an issue.
We used the chemicals; with out the chemicals this lab wouldn’t have been possible.
ROY G BIV is the order of the colors in the spectrum from the lows to the high-test energy.
We used several different chemicals such as ferrous sulfate (FeSO4), cobalt chloride (CoCl2), nickel chloride (NiCl2), monogamous chloride (MnCl2).
All of these materials came handy during the process of the lab.
Put 3-5 drops of the other chemicals into the other chemical and record the results if any.
Ignite the flame slowly with the test tube.
We filled the first row of a microplate with 0% ethanol and labeled it ethanol.
Some of the chemicals were copper chloride, lead nitrate, lithium chloride, and strontium chloride. Those were some of the top chemicals. Some of the other chemicals are sodium chloride, potassium chloride and finally methanol.
This part is most important since with out the chemical there is no lab.
Although the chemicals are not to be put by hand because the hand could catch on fire, so instead a cotton swab and a tonsil is used.
Materials used where flint, Bunsen burner, beaker, Q-tip, tongues and safety goggles because safety is first in Mr. Bigler’s classroom.
Four different kind of reactions occurred in this experiment: single displacement, double displacement, combustion, and no reaction.
The reaction took place because lead and potassium react to the same things, making it impossible for them to react to each other.
The results for Na2SO4 was that it increased, our results, as shown show that only after three rows did it increased, but that shows some sort of an increase, consider that our results could have possible error.
If another chemical did in fact burn the same color, then that chemical does not burn any color while on fire.
The results of this Lab, have proven to be correct, things that could have prevented that from accruing are incorrect use of chemicals, not thoroughly reading the procedure, and also abusing the privilege for the use of fire.
The students followed the procedure carefully with the exception of human error, but to the knowledge of the student no mistakes were made.
There was no chance of making a mistake in the Flame Test Lab, simply because its based on mixing non harmfull chemicals. The mistakes made during our lab were our own mistakes.
It formed that because when the chemical and water had the same amount of drops the solution was at the top, and also the middle of all the other solutions.
Also if your stand or clamp was not tight enough it could fall over and mess your whole experiment up.
The energy was the main part that gave off the energy.
There should be a percent error but you cant tell since some of the colors are different from what they are suppose to be.
Many colors were observed in this lab like red, orange, yellow, purple, green and blue.
It was observed that some chemicals burn alike, unlike others that burn differently.
Every chemical that was used was a huge part of this lab.
Write the wrong color description next to the right chemical name on the chart could be a source of error.
Rolling a ball of a table: when it is rolled off of it, each pair of action force is equal then the force is pushing it up by a force.
It was learned that Chemicals react differently with different chemicals and some chemicals do not even react with each other and when the chemicals react they are balanced.
Although the data shows otherwise there may have been errors made, so there might not have been any pattern at all except for no pattern.
The experiment should be tested again with closer attention to dropping the elements.
The results of this experiment did support the hypothesis made. However, due to probable technical error in performing this experiment, the results did not support the hypothesis.
Based on our knowledge of chromatography, it is inconceivable that our results could have been correct.
The distortion method should also be done so that no water is lost.
All of the substances varied in different ways some more than others, but for all the substances were different.
In the end it was found that a chemical would change its level depending on how much of the chemical or water is in it.
Do to some of the colors could of be doubt with by the fact each student changed stationed right away, but that is not for sure it that is what caused the change in colors or the same.
These colors that were produced could of been may have had causes of other to come out the way they did, but it was not for sure.
The lab could have been made better if some of the elements were closer to one another on the periodic table.
The electron causes the copper ion to change color when it is in heat.
Work Sighted ← heading from a student’s lab report

Love when the copper ion is “in heat”–heheheh! And DNA testing I see, using a cotton swab and tonsil?
Thank you for this list! I laughed a lot. And I needed a good laugh. I’m definitely going to start a list of my own for the gems I come across.